Two theories to explain the cause
of climate change.
Author; Rogelio Pérez C
Summary;
The science that explains temperature in the
atmosphere is governed by the theory of the greenhouse effect, but in science
there is a superior theory to explain the temperature in the atmosphere,
because it is not a theoretical explanation only but also mathematical, and
last and most importantly, it can be experimentally tested on any thermometer,
because these measure the average kinetic energies in the systems, which
confirms the kinetic theory of gases, but the theory of the greenhouse effect
does not have a thermometer that can measure all the infrared that greenhouse
gases such as CO2 accumulate, then its explanation, although widely accepted,
cannot be experimentally verified. In other words, scientists when they show
you the temperature of the planet, what they measure with thermometers is the
average movement of all gas molecules in the atmosphere, regardless of how they
explain it.
Introduction.
There is a problem in the world known as climate
change, which consists of an increase in heat on the planet.
To explain the heat in the atmosphere there are two
scientific theories, but today we only know the greenhouse effect theory, that theoretically explains the
temperature of the atmosphere caused by greenhouse gases, but does not have a
mathematical formula to tell us how this process works, that's why there's no
one to say how many CO2 molecules are needed to increase the temperature of the
atmosphere by 1 degree.
And the second is the kinetic of gases theory, which explains to us theoretically and
mathematically how temperature originates in a gas such as the atmosphere, and
how the kinetic energy of an atom is measured, up to all atoms or molecules
that make up a gaseous system like the atmosphere.
The greenhouse effect theory is due to a great
theorist of science, and winner of a Nobel laureate named Svante August
Arrhenius (1859-1927), among his theory we find the theory "panspermia"
to explain the beginning of life on Earth, Theory of electronic dissociation
which proved to be true only for weak electrolytes, and the greenhouse effect
theory.
The kinetic of gases theory is due to James Clerk
Maxwell (1831-1879), although his greatest achievement was the formulation of
the classical theory of electromagnetic radiation, which first unified
electricity, magnetism and light as distinct manifestations of the same
phenomenon. Maxwell’s equations, formulated for electromagnetism, have been
widely considered the “second great unification of physics,” the first being
that performed by Isaac Newton. Maxwell shares equal importance in science with
newton and Albert Einstein.
science explains heat on the planet based on a theory called greenhouse effect, Which consists in that if a gas is increased in the atmosphere known as CO2, then the temperature of the planet will increase, because the CO2 absorbs infrared, so it can retain infrared, and reemit it in all directions of the atmosphere.
Theory statement and definitions
The greenhouse effect theory
The greenhouse effect is a process in which thermal
radiation emitted by the planetary surface is absorbed by atmospheric
greenhouse gases (GHGs) and radiated in all directions. As part of this
radiation is returned to the Earth's surface and lower atmosphere.1
Greenhouse effect theory 1
Kinetic of gases theory
The kinetic of gases theory is a physical and chemical
theory that explains the macroscopic behavior and properties of gases (the law
of ideal gases), based on a statistical description of microscopic molecular
processes. The kinetic theory was developed based on studies by physicists such
as Daniel Bernoulli in the 18th century, Ludwig Boltzmann and James Clerk
Maxwell in the late 19th century.2
Charles law for gases, for any gas, the ratio between temperature and
volume is directly proportional, if the quantity of gas and pressure remain constant.
Mathematically we can express it like this:
V is the volume
T is the absolute temperature (i.e measured in
kelvin).
k is the constant of proportionality.3
Heat, q, is thermal energy transferred from a hotter
system to a cooler system that are in contact. Temperature is a measure of the
average kinetic energy of the atoms or molecules in the system. The zeroth law
of thermodynamics says that no heat is transferred between two objects in
thermal equilibrium; therefore, they are the same temperature.4
Heat, is thermal energy transferred from a hotter
system to a cooler system that are in contact.
We can calculate the heat released or absorbed using
the specific heat capacity C, the mass of the substance, m, and the change in
temperature, ΔT in the equation:
q=m×C×ΔT
Heat and temperature are two different but closely
related concepts. Note that they have different units: temperature typically
has units of degrees Celsius (degrees °C,) or Kelvin (K), and heat has units of
energy, Joules (J).
Temperature is a measure of the average kinetic energy
of the atoms or molecules in the system. The water molecules in a cup of hot
coffee have a higher average kinetic energy than the water molecules in a cup
of iced tea, which also means they are moving at a higher velocity.5
Temperature is also an intensive property, which means
that the temperature doesn't change no matter how much of a substance you have
(as long as it is all at the same temperature!). This is why chemists can use
the melting point to help identify a pure substance—minus the temperature at
which it melts is a property of the substance with no dependence on the mass of
a sample.
The equipartition theorem relates the temperature of a
system to its average energies. It makes quantitative predictions, provides the
total kinetic and potential energies for a system at a given temperature, from
which the heat capacity of the system can be calculated. However, the
equipartition also provides the average values of individual energy components,
such as the kinetic energy of a particular particle or the potential energy of
a single spring. For example, it predicts that each atom in an ideal monoatomic
gas has an average kinetic energy of (3/2) k B T in thermal equilibrium, where
k B is Boltzmann's constant and Te the temperature (thermodynamics).6
Thermal motion of an α-helical peptide. The jittery
motion is random and complex and the energy of any particular atom can
fluctuate wildly. Nevertheless, the equipartition theorem allows the average
kinetic energy of each atom to be computed, as well as the average potential
energies of many vibrational modes. The grey, red and blue spheres represent
atoms of carbon, oxygen and nitrogen, respectively; the smaller white spheres
represent atoms of hydrogen.7
This work explains the error of
this theory;
first, infrared has nothing to do with heat in the
atmosphere, which is the problem we have, for a simple reason, infrared is
light, and heat is a force of mass, Therefore its unit of measurement is the
Joule and is measured with the following equation; q=m×C×ΔT.
Second, the atmosphere is a system composed of 100% of gas
molecules that radiate infrared in all directions, although 99.9% do not absorb
it, the only fixed gases in the atmosphere that absorb infrared and also
radiate it are greenhouse gases such as CO2, which are only 004% of the atmosphere,
that ends with the deception that because CO2 absorbs infrared is the cause of
emits it in all directions.
Third, the temperature of a gaseous system such as the atmosphere, is explained more than 200 years ago, with the law of gas temperature of the Frenchman Jacques Charles (1746 - 1823), which he said; the temperature of any gas (the atmosphere is a gas) is proportional to its volume, the kinetic theory of gases explains theoretically and mathematically that the temperature of a gas is caused by the kinetic energy of all the molecules that make up this gas, and is measured by the average of this energy, in other words, it is the average of the kinetic movements of all the molecules in a gas (atmosphere).
Conclusión
The main conclusion is that the greenhouse effect theory
has nothing to do with the scientific explanation of temperature and heat in
systems, because it explains the temperature of the atmosphere as caused by the
absorption of infrared radiation by certain gases of the system, and the
temperature of any system originates from the kinetic movement of all the atoms
or molecules that form it, in the atmospheric system are all gas molecules, in
relation to heat, the scientific definition is clear and simple, it is the
transfer of kinetic energy or movements between atoms or molecules of two
systems and its unit of measurement is joule.
The scientific conclusion that the planet has little
time left because of the increase in temperatures, which is based on the science
of the greenhouse effect that governs the climate paradigm, and the greatest
argument for taking measures that affect the inhabitants of the planet, it is
that we have to listen to science, Then I believe that the science of
temperature because of the kinetic energy of gases should also be heard, which
was dictated by one of the greatest
minds of science as James Clerk Maxwell, which is also called by some as the
father of electromagnetic radiation, and it should not be overlooked that the
father of radiation, did not take into account infrared radiation to explain
the temperature of the gases.
Finally whatever theory you like to explain the
temperature of the atmosphere, the apparatus used to measure the temperature of
the systems, are the thermometers and these do not measure the infrared
accumulated in a system, but the kinetic energy of these systems, in other
words these experimentally support the kinetic gases theory. Otherwise there is
no thermometer that experimentally supports the greenhouse effect theory, in
other words, scientists when they show you the temperature of the planet, what
they measure with thermometers is the average movement of all gas molecules in
the atmosphere, regardless of how they explain it.
Bibliography
1- Intergovernmental Panel on Climate Change. Consultado
el 15 de octubre de 2010.
A
concise description of the greenhouse effect is given in the Intergovernmental
Panel on Climate Change Fourth Assessment Report, "What is the Greenhouse
Effect?" FAQ 1.3 - AR4 WGI Chapter 1: Historical Overview of Climate
Change Science, IIPCC Fourth Assessment Report, Chapter 1, page
2- Maxwell, J. C. (1867). "On the Dynamical
Theory of Gases". Philosophical Transactions of the Royal Society of
London 157: 49
3-http://www.educaplus.org/gases/ley_charles.html
4-https://www.khanacademy.org/science/chemistry/thermodynamics-chemistry/internal-energy-sal/a/heat
5-https://www.khanacademy.org/science/chemistry/thermodynamics-chemistry/internal-energy-sal/a/heat
6- http://hyperphysics.phy-astr.gsu.edu/hbase/Kinetic/eqpar.html
7-
https://en.wikipedia.org/wiki/Equipartition_theorem




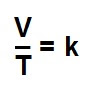

Comentarios
Publicar un comentario